Dear dedicated followers of our cat-filled magnificently informative blog, we, the Beryllium Chemists, would like to give a big THANK YOU for
Without further ado... our last post about organic chemistry (YES) will be on ALICYLICS AND AROMATICS!!
Alicyclics And Aromatics
- Cyclic Hydrocarbons (allicyclics): a ring shaped structure formed by hydrocarbons
- use the prefix "cyclo" in the name
- The general formula is CnH2n
- Obviously, there must be 3 or more carbons to form the ring structure
- When numbering the cyclic hydrocarbons, you may start anywhere and move at anydirection of the ring
- Numbering can be clockwise or counterclockwise for the main chain only!
- They may have branches as well, and be branches
- more reactive than the simpe straight chains
Rules For Naming Branched Cycloalkanes
Now recall your elementary geometry math skills! These are the shapes that alicyclics can form, with each edge of the shape as a hydrocarbon. The number of hydrocarbons (edges) would correspond to the alkane names (propane, butane, hexane.. etc)
1. Count the number of edges (carbons)
2. If there is 1 side group, no numbering is needed
3. If there is more than 1 carbon then the first side group is placed at the number 1 position of the ring (How to choose which is first: lowest numbering, alphabetically)
4. Cyclic substituent is just named like the simple straight chain side group
5. Prefix is "cyclo" and is placed before the main chain name
Cycloalkanes, Cycloalkenes, Cycloalkynes
- Cycloalkenes = double bonds
- Cycloalkynes = triple bonds
- Carbon number 1 and 2 are akways the double or triple bonds
Examples:
cyclobutene cyclooctyne


1-ethyl-3-methylcyclohexane
Notice how we don't start at methyl to produce 3-ethyl-1-methylcyclohexane. Remember that if there are side groups that are equally apart (tied), then start with the alphabetically lower one!
Branched Alicyclics
Basically, alicycylics as side chains have the same rules as any other side groups. The only exception is that there is a "cyclo-" prefix.
In this example, it is called 3-cyclobutylpropene
Aromatics
- Most of the time have a pleasant odour
- Has at least 1 benezene ring (C6H6) with 3 double bonds in between the carbons


- Can be represented with a circle in the middle
- Can be either parent or side chain
- Electrons can share equally and are able to change positions, this is called delocalization
- They are very stable because of delocalization
- Not as reactive as cycloalkenes and cycloalkynes
Aromatic Naming
- "benzene" is the parent chain name
- If it's a side chain, the prefix is "phenyl-"
<--- Ex. 2-chloro-1-iodo-3-methylbenzene
Ex.
2-phenylhexane
Well, it has been a great year serving you guys! So long, and farewell. :)
GOODLUCK STUDYING FOR TESTS AND FINALS and have a great summer!





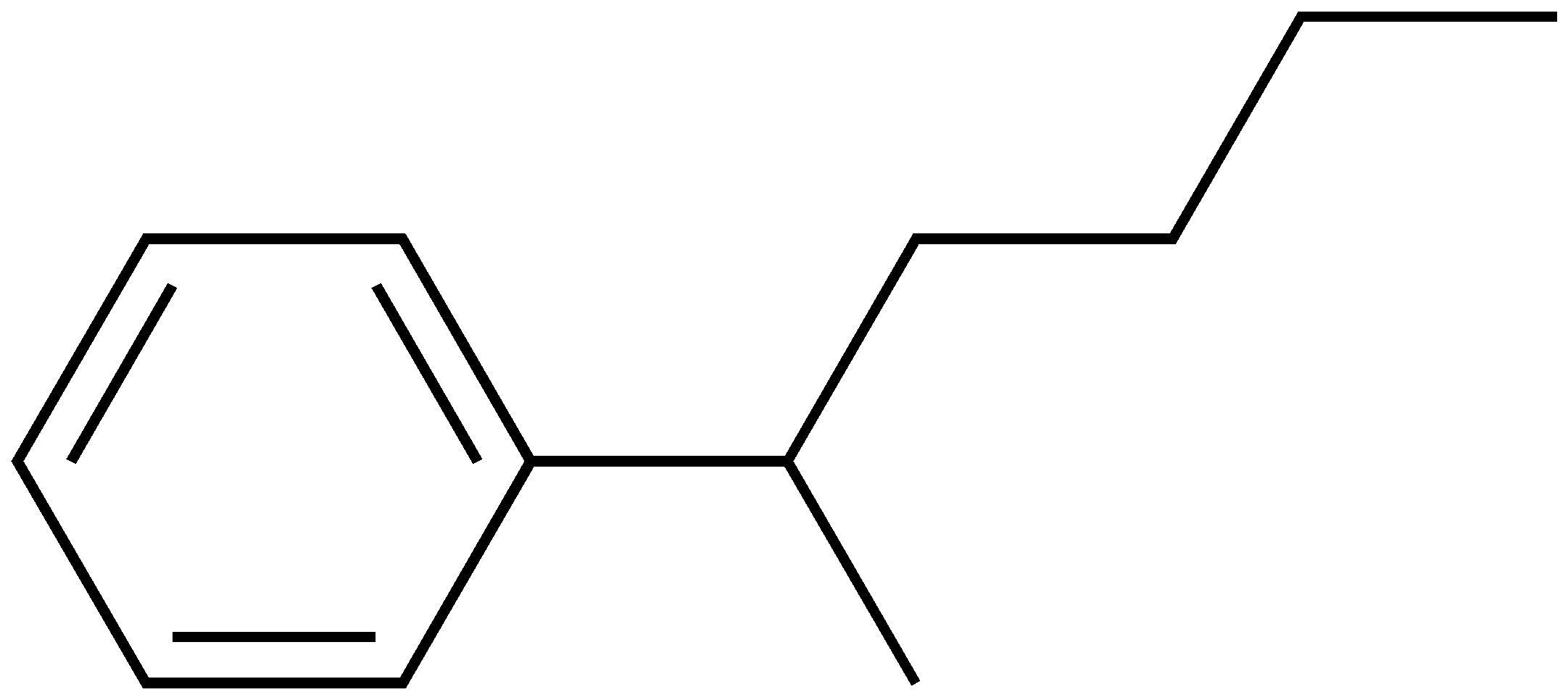



































 hubba hubba ;)
hubba hubba ;)


