So last class, I'm not going to lie, I don't think my brain absorbed anything. I was too busy focussing on copying down the notes as quickly as I could. The good news is, I got almost everything. The bad news - I can barely read my own writing. Cool story bro.
So WHY do we separate...
Well really, because we want the good grade. But don't write that on a test.
We separate substances because we want to view the individual components/properties of each chemical.
So since you remember the basic separation techniques (ha), I don't really need to remind you. But since repetition is supposedly the most effective (and annoying) method of education, I'll list the main techniques out for you. How nice am I.
- Hand Separation (eg using a magnet)- Filtration (separation by particle size)
- Floatation (separating by density)
- Crystallization (separating... by crystallizing a substance)
- Extraction (separating by solubility)
- Extraction (separating by solubility)
- Distillation (separating by boiling point)
- Chromatography (stationary phase)
To start it off, here is a video of some guy (who clearly has no life) separating iron from sand. FYI, this is Hand Separation.
Another way to hand separate is to boil away a liquid, leaving the solid remaining.
Filtration
Filtration is a relatively easy method of separation. It is useful when you have a (non-dissolved) solid and a liquid, and some fancy-schmancy paper. You would pour the mixture into the porous paper, and while the liquid would go right through, the solid would leave a residue on the paper. Magic.
Crystallization
This is a useful procedure when you have a solid in a liquid. The precipitate is 'crysallized,' and then floats because of the change in density. The crystals can then be filtered out.
Floatation (Gravity Separation)
This method is used to separate materials based on their densities. This is done by putting the mixture in a container (most likely test tube) and then swirling it around. IN A CONTROLLED MANNER. You could have an acid in that container, and unless you really dislike your partner and want to splash acid on them, or on yourself (to get away from them, duh), you want to keep the mixture in the container. Plus, you want to see the awesomeness of the separation of densities - while you swirl, gravity will pull the denser substance to the bottom.
Solvent Extraction
Solvent extraction, or just extraction, is the term used for separating two materials based on their solubility (ability to be dissolved) in different solvents.
Distillation
Distillation is a method you could use if you were given two different liquids. You would heat the liquids up, and the liquid with the lower boiling point would vaporize. That leaves you with the other component of the mixture (thank you, captain obvious), and you would be able to collect the volatized (that's a fancy word for vaporized) component later.
Chromatography
We talked about this technique probably more than any other one technique last class. You know, the whole "bees and hornets flying over a flower bed, bees stop, hornets keep going" thingy? Supposedly that's going to help us remember that chromatography is a method where a mixture is flowed over a material that can retain some components of the material. For example, if you mixed honey and water together, theoretically, the honey, which has a higher viscosity than water, would stick to the paper more. Meanwhile, the water would flow right on by. Like I said, that is my hypothesis. I'm not wasting any perfectly good honey to test that out.
Heating Curve
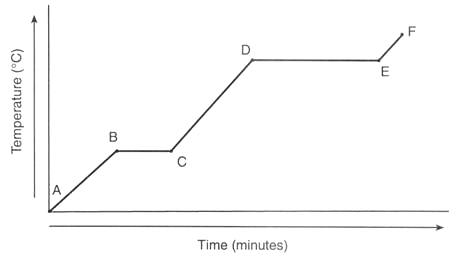 A: Solid; closely packed in an orderly manner, strong bonds and vibrates at a fixed position
A: Solid; closely packed in an orderly manner, strong bonds and vibrates at a fixed position
A→B: Still a solid; heat is converted to kinetic energy, vibrates a bit faster, KE and temperature increases
B: Same or similar to "A"
B→C: Melting point (solid -> liquid); heat absorbed is called latent heat of fusion, temp stays the same because heat is used to overcome forces of attraction that hold particles together
C: Liquid; solid has melted
C→D: Temperature and KE increases, particles move faster
D: Still liquid but starts changing from liquid state to gas; some molecules start to move freely
D →E: Boiling point (liquid -> gas); temperature stays the same since heat is used to overcome the forces of attraction that hold the particles together
E: Gas state
E→F: Heating continues so KE and temp increases; particles move faster
Cooling Curve

P: Gas; particles have more high energy and moves quickly
P→Q: Temperature and KE decreases; particles are getting closer together
Q: Still a gas; start to form intermolecular bonds, condensation begins, starts to form a liquid
Q→R: Boiling point (gas to liquid); condensation, temperature is constant, heat energy is released called latent heat of vaporization
R: Liquid state
R→S: Temperature and KE decreases; molecules lose energy, vibrate slower and moves closer to each other
S: Starts to freeze into a solid
S→T: Freezing point (liquid to solid); particles arrange in an ordered manner
T: Solid state
T→U: Temperature decreases until room temperature
U: Substances has reached room temperature:
U→V: Remains as a solid in room temp.
Heating Curve
 A: Solid; closely packed in an orderly manner, strong bonds and vibrates at a fixed position
A: Solid; closely packed in an orderly manner, strong bonds and vibrates at a fixed positionA→B: Still a solid; heat is converted to kinetic energy, vibrates a bit faster, KE and temperature increases
B: Same or similar to "A"
B→C: Melting point (solid -> liquid); heat absorbed is called latent heat of fusion, temp stays the same because heat is used to overcome forces of attraction that hold particles together
C: Liquid; solid has melted
C→D: Temperature and KE increases, particles move faster
D: Still liquid but starts changing from liquid state to gas; some molecules start to move freely
D →E: Boiling point (liquid -> gas); temperature stays the same since heat is used to overcome the forces of attraction that hold the particles together
E: Gas state
E→F: Heating continues so KE and temp increases; particles move faster
Cooling Curve

P: Gas; particles have more high energy and moves quickly
P→Q: Temperature and KE decreases; particles are getting closer together
Q: Still a gas; start to form intermolecular bonds, condensation begins, starts to form a liquid
Q→R: Boiling point (gas to liquid); condensation, temperature is constant, heat energy is released called latent heat of vaporization
R: Liquid state
R→S: Temperature and KE decreases; molecules lose energy, vibrate slower and moves closer to each other
S: Starts to freeze into a solid
S→T: Freezing point (liquid to solid); particles arrange in an ordered manner
T: Solid state
T→U: Temperature decreases until room temperature
U: Substances has reached room temperature:
U→V: Remains as a solid in room temp.
Well, thanks for not reading this. I'm just gonna remind myself that I better go do that Pre-Lab homework. Can I get a whoot whoot?
Nope. Didn't think so.
XOXO BerylliumChemists
(written by Heather LeWonderful)



No comments:
Post a Comment