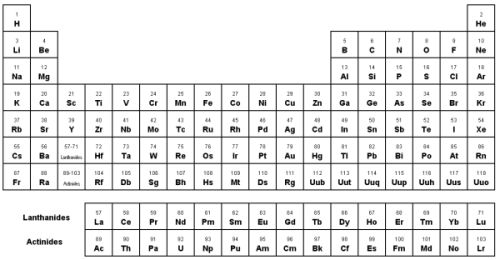
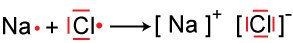| Group | ||||||||||||||||||
| Period | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| 1 | H 2.1 | He 0 | ||||||||||||||||
| 2 | Li 0.98 | Be 1.57 | B 2.04 | C 2.55 | N 3.04 | O 3.44 | F 3.98 | Ne 0 | ||||||||||
| 3 | Na 0.93 | Mg 1.31 | Al 1.61 | Si 1.9 | P 2.19 | S 2.58 | Cl 3.16 | Ar 0 | ||||||||||
| 4 | K 0.82 | Ca 1 | Sc 1.36 | Ti 1.54 | V 1.63 | Cr 1.66 | Mn 1.55 | Fe 1.83 | Co 1.88 | Ni 1.91 | Cu 1.9 | Zn 1.65 | Ga 1.81 | Ge 2.01 | As 2.18 | Se 2.55 | Br 2.96 | Kr 0 |
| 5 | Rb 0.82 | Sr 0.95 | Y 1.22 | Zr 1.33 | Nb 1.6 | Mo 2.16 | Tc 1.9 | Ru 2.2 | Rh 2.28 | Pd 2.2 | Ag 1.93 | Cd 1.69 | In 1.78 | Sn 1.96 | Sb 2.05 | Te 2.1 | I 2.66 | Xe 2.6 |
| 6 | Cs 0.79 | Ba 0.89 | La 1.1 | Hf 1.3 | Ta 1.5 | W 2.36 | Re 1.9 | Os 2.2 | Ir 2.2 | Pt 2.28 | Au 2.54 | Hg 2 | Tl 2.04 | Pb 2.33 | Bi 2.02 | Po 2 | At 2.2 | Rn 0 |
| 7 | Fr 0.7 | Ra 0.89 | Ac 1.1 | Rf | Db | Sg | Bh | Hs | Mt | Uun | Uuu | Uub | ||||||
| Lanthanides | Ce 1.12 | Pr 1.13 | Nd 1.14 | Pm 1.13 | Sm 1.17 | Eu 1.2 | Gd 1.2 | Tb 1.1 | Dy 1.22 | Ho 1.23 | Er 1.24 | Tm 1.25 | Yb 1.1 | Lu 1.27 | ||||
| Actinides | Th 1.3 | Pa 1.5 | U 1.38 | Np 1.36 | Pu 1.28 | Am 1.3 | Cm 1.3 | Bk 1.3 | Cf 1.3 | Es 1.3 | Fm 1.3 | Md 1.3 | No 1.3 | Lr | ||||
Chemical bonds are formed when the electrons of one atom are attracted by the nucleus of another atom. They are also formed with various degrees of sharing of electrons between two atoms.
RECALL:
RECALL:
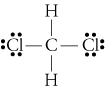
If the electron are shared equally between the two atoms, it is called a COVALENT BOND.
If the electron of one atom are given away completely to another atom, oppositely charged ion are then held together through an electrostatic attraction, and the force holding the two ions together is called an IONIC BOND.
There are various degrees of sharing which goes on between two atoms, depending on the amount of attraction each atom has for receiving another atom.
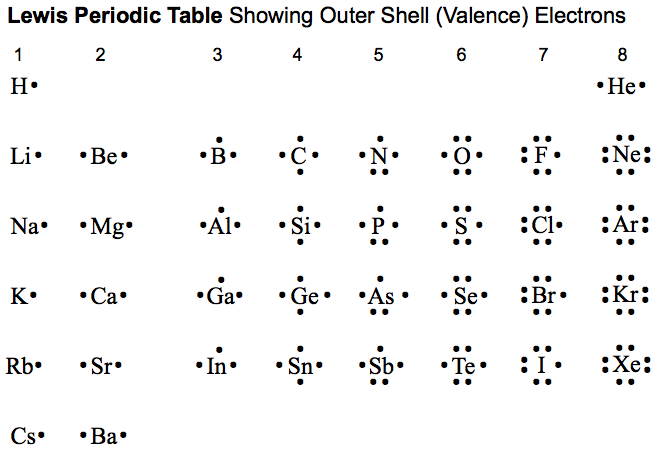
The attraction an atom has for the shared pair of electrons in a chemical bond is called an atom's ELECTRONEGATIVITY. It is the difference in electronegativity between two atoms that determines the degree of electron sharing which occurs between the two atoms.
Some fun facts:
- the atom with the highest electronegativity on the periodic table is Fluorine. It has a value of 4.0. This high value indicates that the atom readily pulls on electrons in a bond.
- the atom with the lowest electronegativity on the periodic table is Francium. It has a value of 0.7. This low value indicates that it doesn't readily pull on electrons within a bond.
And the answer is... well it probably isn't very important. But we need to know it because it helps us determine the polarity of a compound.
Remember, there are 2 type of covalent bonds: non-polar and polar.
You know you have a non-polar covalent bond if the difference between the electronegativities of the two elements comprising the bond have a difference of less than 0.5.
If the electronegativity difference is between 0.5 and 1.8, then it is a POLAR covalent bond.
Once in a while, you get these weird situations where you have a metal and a non-metal bonding that has an electronegativity difference of less than 1.8, and you're wondering WHAT THE HECK MY TEACHERS HAVE BEEN LYING TO ME ABOUT IONIC BONDS. I thought this too. But actually, it's just the electronegativity difference that makes them seem not ionic. They are still ionic compounds because they bond by transferring electrons, unlike the typical sharing of covalent bonds.

When you have a compound, you may be asked to draw out a diagram showing the movement of electrons (the electron shift).
When you have a compound where there is a shift in electrons, that means one element gave away an electron and the other element gained one. That leads to the conclusion that the element that gave away the electron becomes partially positive [it lost a -1 charge --> -(-1) = +1], and the element that gained an electron becomes partially negative.
For example... As you can see below, I kindly drew out for you what Lithium and Flourine would look like. Since Fluorine has 7 valence electrons, it desperately wants 1 more to fill its shell. On the other hand, Lithium only has 1 valence electron that it is dying to get rid of. So Lithium kindly donates its electron to the cause, and allows Flourine to gain its full valence shell. And everybody's happy! yay! So since Lithium gave away an electron, it becomes partially positive. That squiggly S like thing means partially. Cool beans.
When you have a compound where there is a shift in electrons, that means one element gave away an electron and the other element gained one. That leads to the conclusion that the element that gave away the electron becomes partially positive [it lost a -1 charge --> -(-1) = +1], and the element that gained an electron becomes partially negative.
For example... As you can see below, I kindly drew out for you what Lithium and Flourine would look like. Since Fluorine has 7 valence electrons, it desperately wants 1 more to fill its shell. On the other hand, Lithium only has 1 valence electron that it is dying to get rid of. So Lithium kindly donates its electron to the cause, and allows Flourine to gain its full valence shell. And everybody's happy! yay! So since Lithium gave away an electron, it becomes partially positive. That squiggly S like thing means partially. Cool beans.