It's actually this guy...

Kidding again!
Another joke of the day:
Why did Carbon marry Hydrogen?
I don't know, why?
They bonded well from the minute they met. HAH
So today we will be talking about BONDING! Specifically, chemical bonding, which involves the transfer and sharing of valence electrons to gain stability (closed shell).
There's an existing force of attraction or repulsion that exists between two charged particles and that is the electrostatic force. This force is acted upon in all directions.
Few things to note on:
- Opposite charges attract, and like charges repel
- The greater the distance, the smaller the attractive force
- The greater the charge, the greater the attractive force
Ionic Bonds
- the transfer of valence electrons from one atom to another
- formed by attraction of a metal to a non-metal
- creates a positive and a negative ion
Atoms lose or gain enough electrons to attain a closed cell
Ionic bonds are arranged in a crystal lattice structure where there is an equal number of alternating positive and negative charges
Example of NaCl:
Ionic bonds are very strong, and only an enormous of energy can break the bonds. So if they need a LOT of energy, then the melting point must be high.
Electronegativity: measure of tendency of an atom to attract electrons from a neighbouring atom
(Non-Polar) Covalent Bonding
Non-polar covalent bonding is based on the octet rule. That is, non-metals share electrons in order to obtain a closed shell. Wow sharing?! If my sister and I were a bond, we would be ionic. As in I would steal her electrons. We don't share, as a rule. Sharing means I take stuff, say I will share, and then don't. She usually forgets, so it's a very effective method of winning. Elements have a lot to learn from me.
Here is an example of a covalent bond:
The electrons are shared equally among themselves, and each atom satisfies the octet rule. This nonpolar covalent bond is symmetrical, which is another factor of determining what type of covalent bond it is (we'll get to that later).
Anyways, I'm sorta like a non-metal, since non-metals have high levels of electronegativity, which means they attract electrons very strongly. But, that being said, none of them are up to giving away any. Greedy? I think so. So these non-metals have come up with a bit of a compromise - they share electrons with other non-metals in order for both elements to be satisfied. See, that's where humans (me...) and elements differ. Elements are willing to share electrons to achieve a win-win, but for humans - a win isn't a WIN unless there's a loser. Just saying.
IMPORTANTTERMSTHATYOUSHOULDNOTFORGET
omg i held the shift button for that entire thing instead of using caps lock... dummy.
INTRAmolecular forces (aka covalent bond) - found in the INTERIOR of the molecule - holds the molecule together
and the weaker INTERmolecular forces - found BETWEEN molecules, and holds 2 SEPARATE molecules together
example:
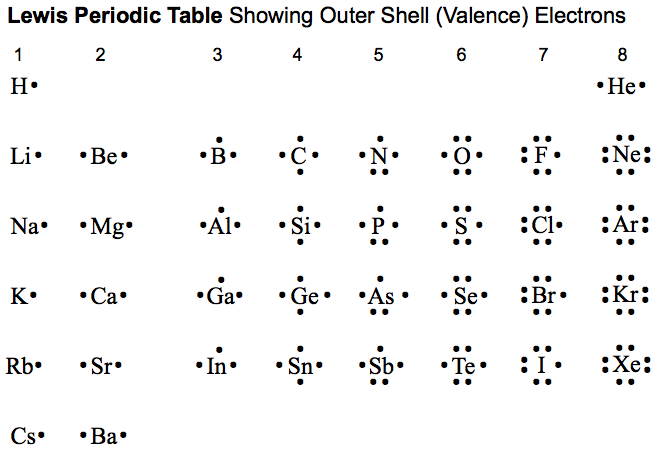
Drawing Lewis Dot Structures
Here's a chart that shows the Lewis dot structures for main elements that we will use this year. The Lewis dot structures only show the number of valence electrons. Remember to follow the octet rule when bonding! (atoms tend to lose/gain and share electrons until they are stable)
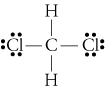
Covalent bonds SHARE electrons, so thus they are bonded like the diagram below.
Example: Hydrogen has one valence electron and Chlorine has 7. When they share their unpaired electrons, each of the atoms form a stable shell.
Note that the horizontal line represents the covalent bond (the sharing of electrons).
For covalent bonds, there can be more than one bond. Sometimes, when an atom has more bonding electrons - which are unpaired electrons - it can form double to triple bonds.
IONIC:
Ionic bonds transfer their electrons, thus a metal will give away their electrons and the non-metals will then gain them.
Example: Sodium has one valence electron to give away to Chlorine, who needs one more to become stable.
SO. We have a test on Tuesday the 24th. Do not forget or you will fail. Do not be THAT PERSON who walks in asking why everyone is studying and then has a minor heart attack and faints when they discover there is a test.
Yes, you. There's always gotta be someone...









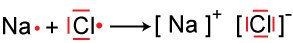
No comments:
Post a Comment